| CAS Number | 437-38-7 |
|---|---|
| Molecular Formula | C22H28N2O |
| Molecular Weight | 336.480 |
| InChI Key | PJMPHNIQZUBGLI-UHFFFAOYSA-N |
| LogP | 4.05 |
| Synonyms |
|
Applications:
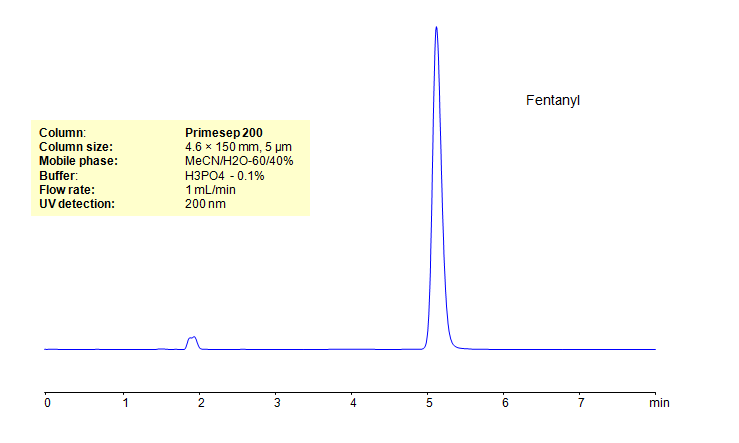
HPLC Determination of Fentanyl on Primesep 200 Column
March 29, 2019
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | H3PO4 – 0.1 % |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of compounds |
Narcotic, Drug, Basic, Hydrophobic, Ionizable, Zwitterionic |
| Analyzing Compounds | Fentanyl |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsFentanyl citrate

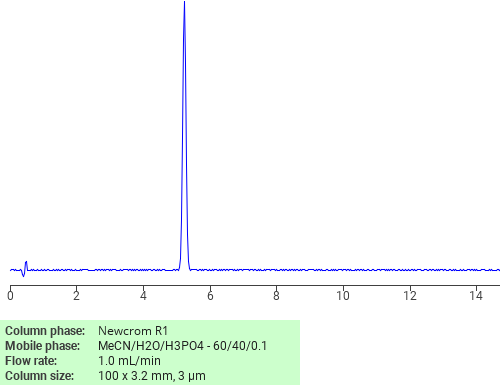
Separation of Fentanyl on Newcrom R1 HPLC column
May 16, 2018
Fentanyl can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options