| CAS Number | 58-63-9 |
|---|---|
| Molecular Formula | C10H12N4O5 |
| Molecular Weight | 268.230 |
| InChI Key | UGQMRVRMYYASKQ-KQYNXXCUSA-N |
| LogP | -2.10 |
| Synonyms |
|
Applications:
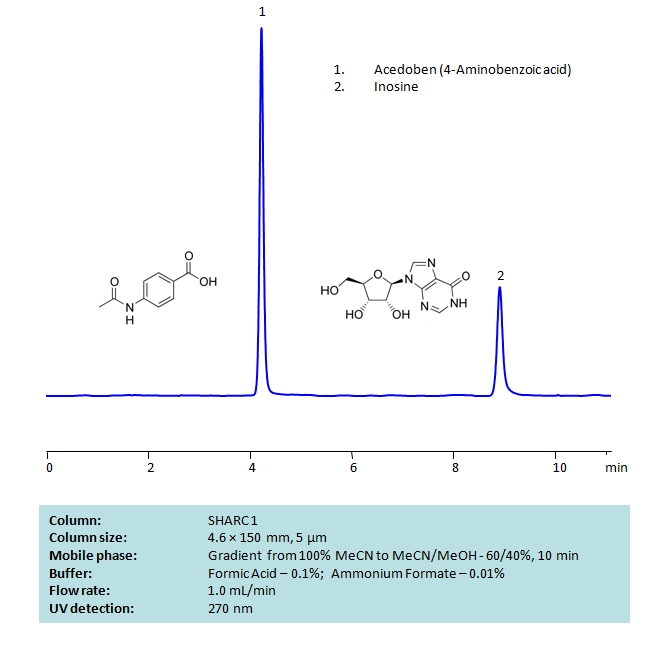
HPLC Separation of Acedoben and Inosine on SHARC 1 Column
December 21, 2020
Inosine pranobex is an antiviral drug. A combination of inosine and dimepranol acedoben (a salt of acetamidobenzoic acid and dimethylaminoisopropanol) has no effect on viral particles itself. Instead, it acts as an immunostimulant. It is most commonly used to treat the rare measles complication subacute sclerosing panencephalitis in conjunction with intrathecal interferon therapy.
Chromatography of these two compounds can be difficult due to their high polarity. But both compounds can be well retained and separated using anhydrous (water-free) conditions using HPLC on SHARC 1 column, which uses hydrogen-bonding as a separation mechanism. The method uses a gradient of acetonitrile (ACN) and methanol (MeOH) mobile phase with volatile buffer containing Formic Acid 0.1% and AmFm – 0.01%, making the method MS-compatible. Both compounds can also be UV detected at 270 nm.
| Column | Sharc 1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | Formic Acid 0.1% , AmFm – 0.01% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Nucleoside monophosphate, Hydrophilic, Ionizable |
| Analyzing Compounds | Inosine, Acedoben |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsInosine

HPLC Analysis of Inosine
August 6, 2015
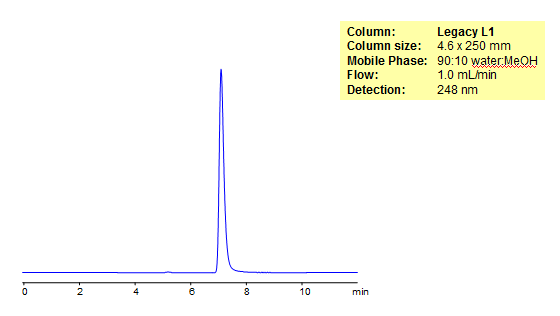
Inosine is a nucleoside made of a hypoxanthine and a ribose ring. As a drug, Inosine is useful for the treatment of various autoimmune diseases. Legacy L1 was used to retain Inosine by reverse phase mechanism. Legacy L1 uses embedded C18 groups on porous silica and is useful for many USP HPLC applications. comparisons to Phenomenex columns are available by request.
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeOH – 10% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 248 nm |
| Class of Compounds |
Nucleoside, Hydrophilic, Ionizable |
| Analyzing Compounds | Inosine |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
HPLC Separation of Inosine and Deoxyinosine
July 11, 2012
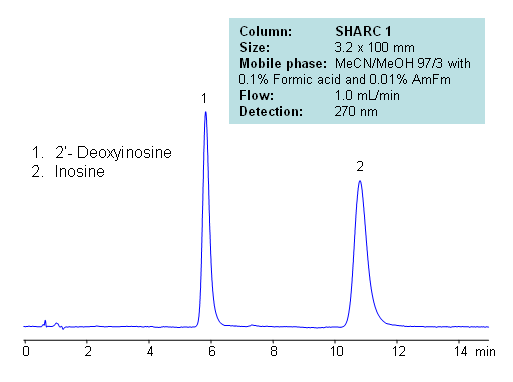
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH – 97/3% |
| Buffer | Formic Acid 0.1% , AmFm – 0.01% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Nucleoside monophosphate, Hydrophilic, Ionizable |
| Analyzing Compounds | Inosine, Deoxyinosine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsInosine