| CAS Number | 55-18-5 |
|---|---|
| Molecular Formula | C4H10N2O |
| Molecular Weight | 102.137 |
| InChI Key | WBNQDOYYEUMPFS-UHFFFAOYSA-N |
| LogP | 0.5 |
| Synonyms |
|
Applications:
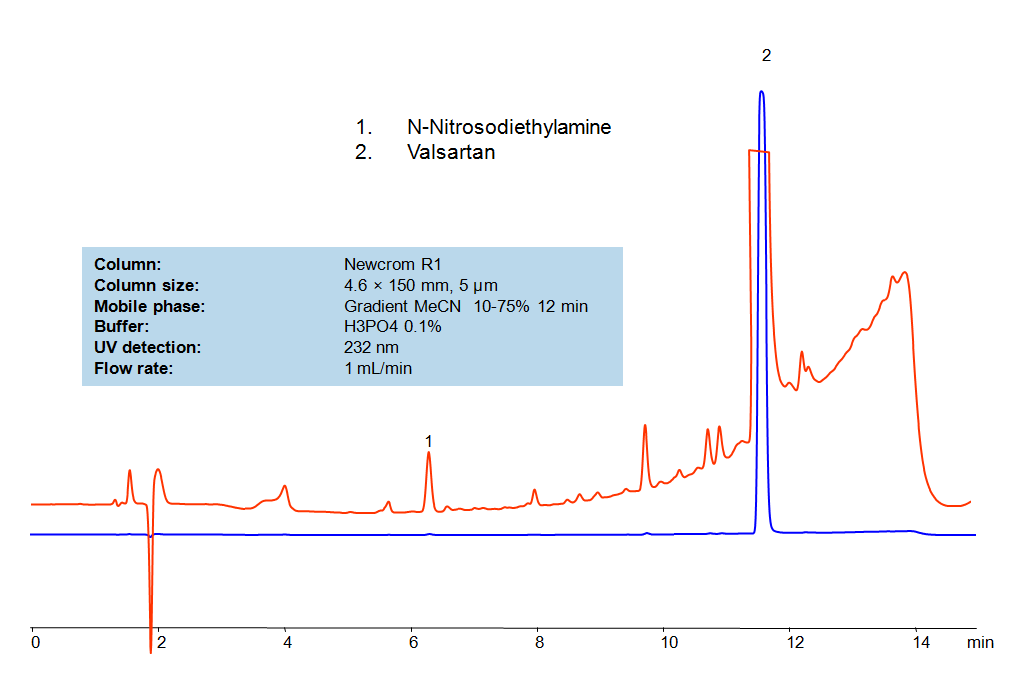
HPLC Method for Detection of NDEA in Valsartan Drug Substance
November 14, 2018
Valsartan products are used to treat high blood pressure and congestive heart failure. On July 13, 2018, FDA announced a recall of valsartan tablets because of the potential for certain products to contain an impurity, N-nitrosodimethylamine (NDMA). Nitrosamine impurities is classified as a probable human carcinogen and is believed to have been introduced into the finished products as a result of the manufacturing process of the drug substance.
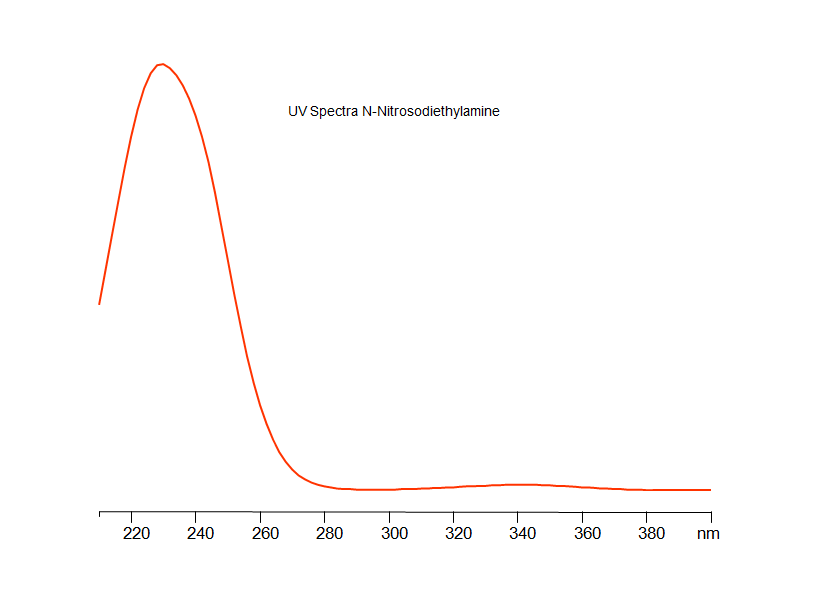
SIELC developed simple and fast HPLC method to measure presence of NDEA in drug formulations. This UV based method allows to detect ppb level of the compound in solution. Simple mobile phase comprised of acetonitrile (MeCN) and water with detection at 232 nm using Newcrom R1 column can be used to detect both the API and the NDEA.
| Column | Newcrom R1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-75%, 12 min |
| Buffer | H3PO4- 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 232 nm |
| Class of Compounds |
Drug, Carcinogen, Zwitterionic, Hydrophilic, Hydrophobic, Ionizable |
| Analyzing Compounds | NDEA, Valsartan |
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsValsartan

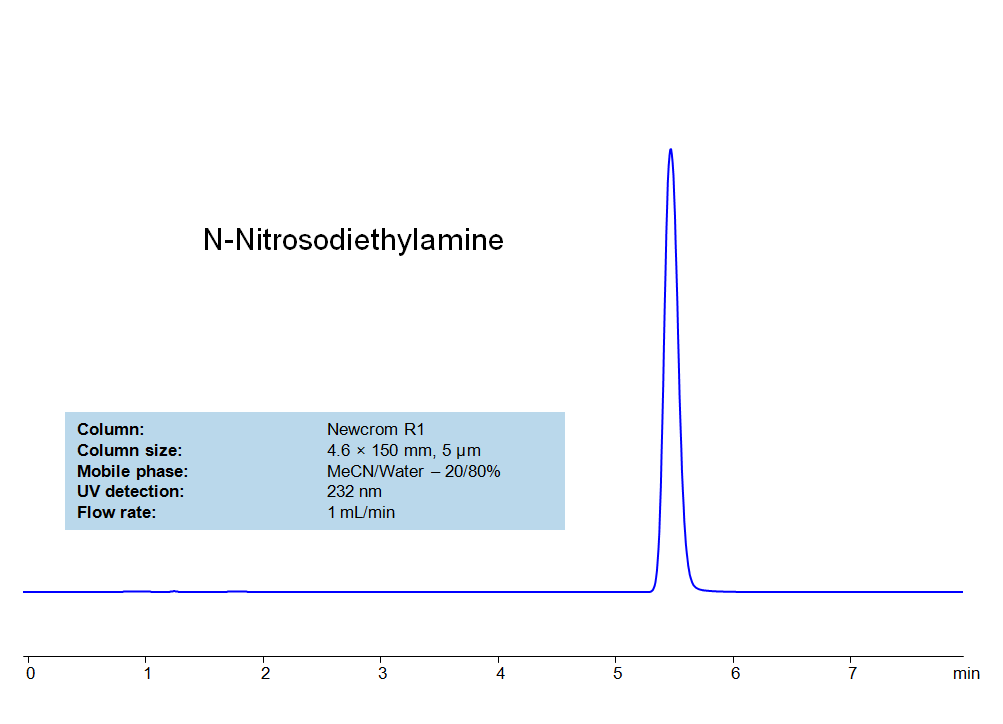
HPLC Method for Analysis of N-Nitrosodiethylamine on Newcrom R1 Column
November 14, 2018
N-Nitrosodiethylamine (NDEA) or Diethylnitrosamine is potential carcenogenic agent was found in some blood pressure medications. Those medications were recalled by FDA with the concern of high concentration of the chemical.
SIELC developed simple and fast HPLC method to measure presence of NDEA in drug formulations. This UV based method allows to detect ppb level of the compound in solution. Simple mobile phase comprised of acetonitrile (MeCN) and water with detection at 2** nm using Newcrom R1 column can be used to detect both the API and the NDEA.
| Column | Newcrom R1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 20% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 232 nm |
| Class of Compounds | Carcinogen, Zwitterionic, Hydrophobic |
| Analyzing Compounds | NDMA |
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options