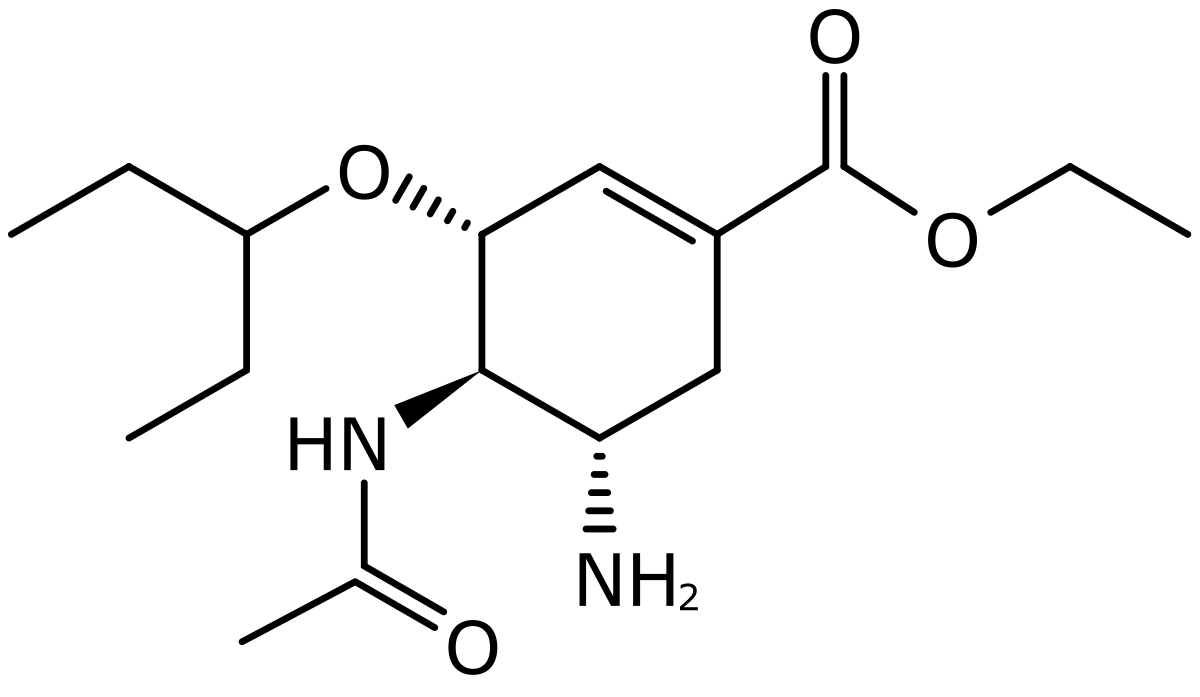
| CAS Number | 196618-13-0 |
|---|---|
| Molecular Formula | C16H28N2O4 |
| Molecular Weight | 312.4 |
| InChI Key | VSZGPKBBMSAYNT-RRFJBIMHSA-N |
| LogP | 1.1 |
| Synonyms |
|
Applications:
HPLC Separation of Antiviral Drugs on Primesep 100 Column
April 1, 2021
Separation type: Liquid Chromatography Mixed-mode
![]()
View on hplc.cloud
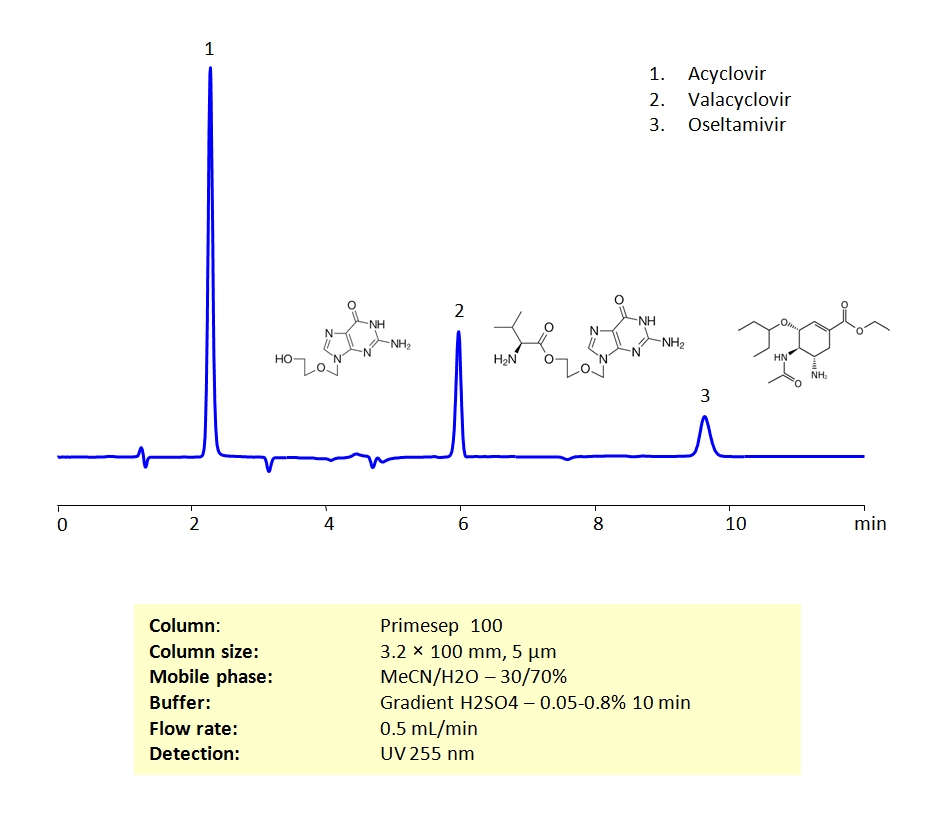
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acyclovir, Oseltamivir, Valacyclovir
Unlike antibiotic drugs, which actively destroy their targets, antiviral drugs generally prevent the reproduction of their viral targets. Acyclovir, Valacyclovir, and Oseltamivir are 3 antiviral drugs commonly prescribed to treat viral infections. Acyclovir and Valacyclovir are used to treat viral infections caused by a group of viruses called herpes simplex viruses. These infections include oral and genital herpes, as well as shingles. They can also be used to treat chickenpox in children. Oseltamivir is generally used to help treat (or if taken early enough, prevent) influenza (the flu) infections.
Acyclovir, Valacyclovir, and Oseltamivir can be retained on a Primesep 100 mixed-mode column with great peak shape using acetonitrile (ACN), water and a gradient of sulphuric acid (H2SO4) as a buffer. This method can be UV detected at 200 nm with very high resolution.
| Column | Primesep 100, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | Gradient H2SO4 – 0.05- 0.8%, 10 min |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 255 nm |
| Class of Compounds |
Drug |
| Analyzing Compounds | Acyclovir, Valacyclovir, Oseltamivir |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsOseltamivir
Valacyclovir

HPLC Determination of Oseltamivir on Primesep 100 Column
March 31, 2021
Separation type: Liquid Chromatography Mixed-mode
![]()
View on hplc.cloud
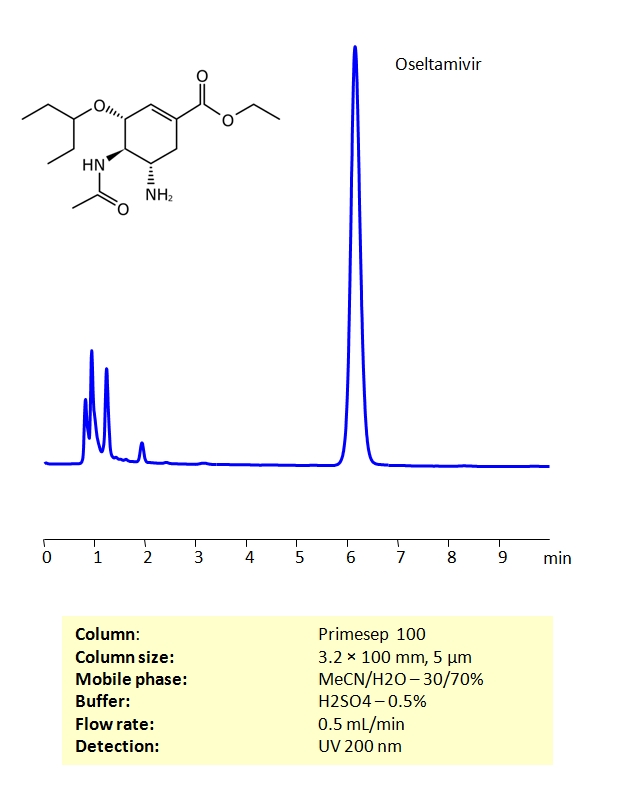
High Performance Liquid Chromatography (HPLC) Method for Analysis of Oseltamivir
Oseltamivir belongs to a class of drugs called antivirals, which are used to treat infections caused by viruses. Oseltamivir is used in the treatment of the infection caused by the flu virus (influenza A and influenza B). Oseltamivir may also be used to prevent and treat swine influenza A. Valacyclovir can be retained on a Primesep 100 column with great peak shape using an isocratic method of acetonitrile (ACN), water and sulphuric acid (H2SO4) as a buffer. UV Detection 200nm.
| Column | Primesep 100, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H2SO4 – 0.5% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug |
| Analyzing Compounds | Oseltamivir |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options