| CAS Number | 61-76-7 |
|---|---|
| Molecular Formula | C9H14ClNO2 |
| Molecular Weight | 203.670 |
| InChI Key | OCYSGIYOVXAGKQ-FVGYRXGTSA-N |
| LogP | -0.204 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Phenylephrine HCl on Primesep S Column by SIELC Technologies
September 26, 2022
HPLC Method for Analysis of Phenylephrine HCl on Primesep S Column
Separation type: Liquid Chromatography Mixed-mode
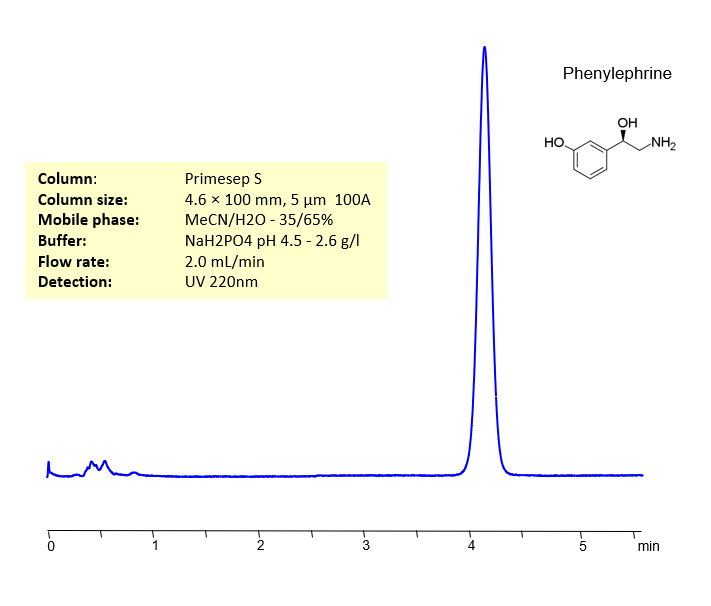
Phenylephrine HCl is a fairly popular decongestant sold under many brand names including Mucinex, Sudafed PE, Sinex, and many other generic brands. While it is best known as a decongestant, it has a wide variety of other pharmaceutical applications, including pupil dilation, hypotension (low blood pressure) treatment, and hemorrhoid relief. Phenylephrine HCl can be detected in the low UV regime. Using a Primesep S normal-phase column and a mobile phase consisting of water and Acetonitrile (MeCN) with a Monosodium Phosphate (NaH2PO4) buffer, Phenylephrine HCl can be retained and analyzed. This analysis method can be UV detected at 220 nm.
| Column | Primesep S, 4.6×100 mm, 3 µm, 100A |
| Mobile Phase | MeCN – 35% |
| Buffer | NaH2PO4 pH 4.5 – 2.6 g/l |
| Flow Rate | 2.0 ml/min |
| Detection | UV 220 nm |
| Retention Time | 4.12 min |
| Class of Compounds | Drug |
| Analyzing Compounds | Phenylephrine |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
HPLC Method for Analysis of Neurotransmitters Phenylephrine, Epinephrine and Norepinephrine on BIST™B+ Column
July 14, 2022
| Separation type: Bridge Ion Separation Technology, or BIST™ | ||||||||||||||
| BIST™ Ionic Modifier Preparation | ||||||||||||||
| High Performance Liquid Chromatography (HPLC) Method for Analysis of Phenylephrine, Epinephrine, Norepinephrine
Epinephrine and norepinephrine are key neurotransmitters and adrenal hormones naturally produced by the body to generate the fight-or-flight response, and synthesized by pharmaceutical companies to treat a host of issues, such as allergic reactions and low blood pressure. Phenylephrine is a related, synthetic compound made to treat low blood pressure, congestion, and hemorrhoids. Using SIELC’s newly introduced BIST™ method, these three similar neurotransmitters, which protonate in water, can be retained on a positively-charged anion-exchange BIST™ B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively-charged amine analytes to the positively-charged column surface and 2) a mobile phase consisting mostly of organic solvent to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Epinephrine, norepinephrine, and phenylephrine can be retained and UV detected at 200 nm.
|
||||||||||||||
|
Application Column
BIST B+
BIST™ columns offer a unique and effective way to achieve separations that were traditionally challenging or even impossible with other HPLC columns. With the use of a special mobile phase, these ion exchange columns provide very strong retention for analytes with the same charge polarity as the stationary phase, unlocking new chromatography applications. What makes BIST™ columns stand out is their proprietary surface chemistry, which results in superior selectivity, resolution, and sensitivity. These columns offer a simple, efficient solution for a variety of analytical challenges, making them an excellent choice for researchers and analysts across many different fields. To learn more about the technology that powers BIST™ columns and to explore related applications, check out https://BIST.LC.
Select optionsNorphenylephrine
Phenylephrine
Phenylephrine hydrochloride

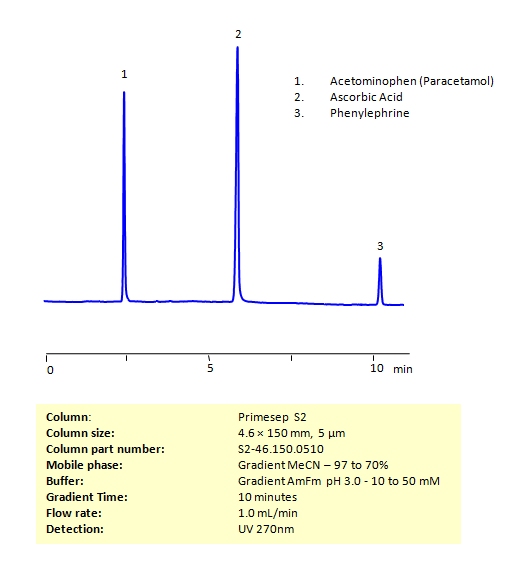
HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S2 Column
October 5, 2021
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
Acetaminophen (or paracetamol) is one of the most popular over-the-counter painkillers all over the world (in the US it is best known under the brand name Tylenol). Ascorbic acid (also known as Vitamin C) helps your body build up muscle, cartilage, and other important building blocks in your body. Phenylephrine is a common over-the-counter decongestant, but can also be used for pupil dilation and hemorrhoid treatment. While all of these compounds are available at your local pharmacy, Vitamin C intake can reduce the body’s ability to break down acetaminophen.
All three of these compounds can be measured at low UV. Using a Primsep S normal phase column and a mobile phase consisting of water and acetonitrile (MeCN) with an Ammonium acetate (AmAc) buffer, these three compounds can be separated and UV detected at 270 nm. Varying the buffer concentration changes the order in which phenylephrine and ascorbic acid are retained. This method is compatible with Mass Spectrometry.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 97 to 70% |
| Buffer | Gradient Ammonium Formate pH 3.0 – 10 to 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm MS- compatible mobile phase |
| Class of Compounds | Drug, Acid |
| Analyzing Compounds | Acetaminophen, Phenylephrine, and Ascorbic Acid |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAscorbic Acid
Phenylephrine
Phenylephrine hydrochloride

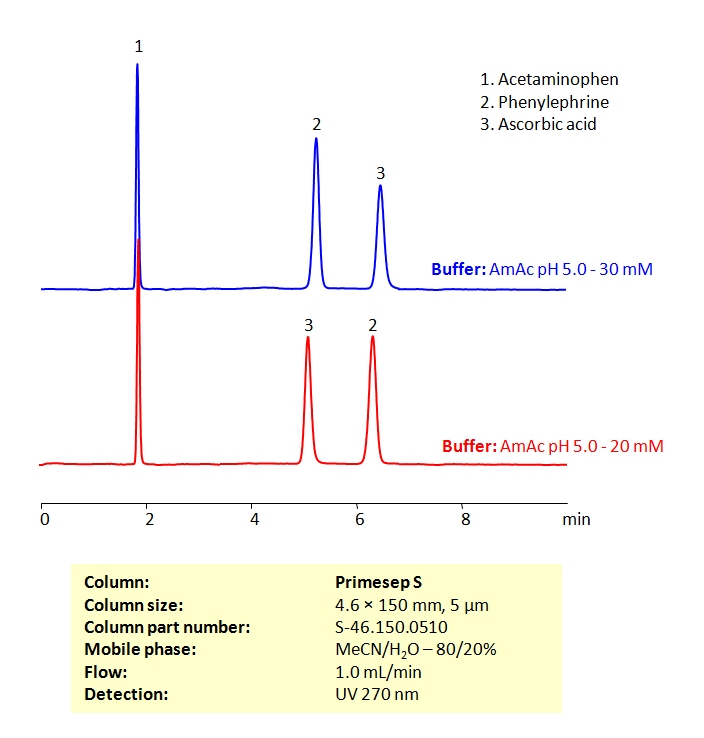
HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S Column
October 5, 2021
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
| Column | Primesep S, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | Ammonium acetate pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270nm MS- compatible mobile phase |
| Class of Compounds | Drug |
| Analyzing Compounds | Acetaminophen, Phenylephrine, and Ascorbic Acid |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAscorbic Acid
Phenylephrine
Phenylephrine hydrochloride

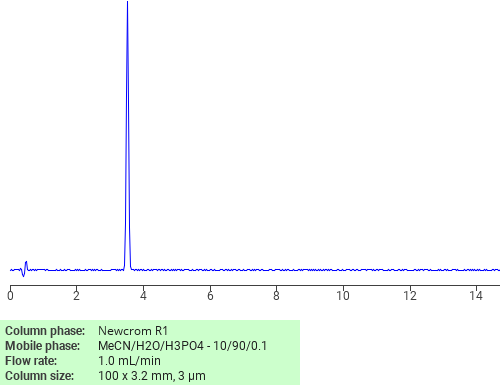
Separation of Phenylephrine hydrochloride on Newcrom R1 HPLC column
February 16, 2018
Phenylephrine hydrochloride can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options