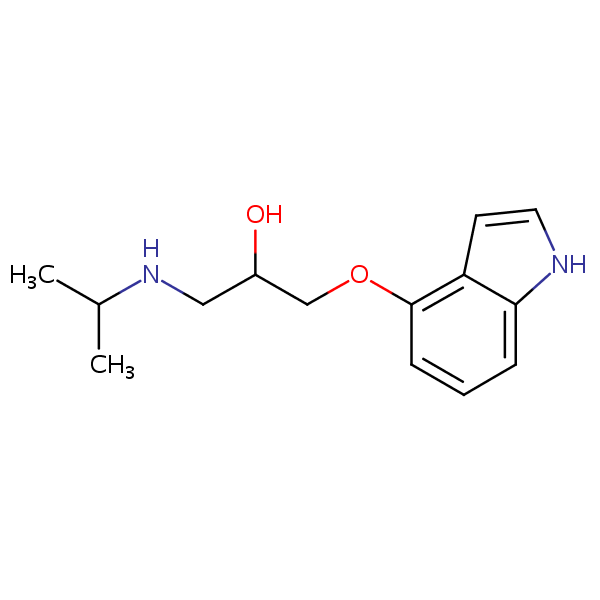
| CAS Number | 13523-86-9 |
|---|---|
| Molecular Formula | C14H20N2O2 |
| Molecular Weight | 248.327 |
| InChI Key | JZQKKSLKJUAGIC-UHFFFAOYSA-N |
| LogP | 1.75 |
| Synonyms |
|
Applications:
HPLC Separation of Pyrilamine, Trimipramine, Pindolol Using Hydrogen Bonding Mode
June 15, 2012

Application Notes: Many drugs contain small hydrophobic and hydrophilic compounds. There are several ways to retain and analyze these compounds including, reversed-phase chromatography, cation-exchange chromatography, and HILIC. Our method includes separation based on hydrogen-bonding interactions between the analytes and the stationary phase. Hydrogen bonding offers unique selectivity of separation with good peak shape and retention control. Our method is fully compatible with ELSD, LC/MS and preparative chromatography. This approach can also be applied to the analysis of other drug molecules.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A. To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Pyrilamine, trimipramine, and pindolol
Detection Technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Pyrilamine, Trimipramine, Pindolol |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsPyrilamine