Pralidoxime chloride has its most critical effect in relieving paralysis of the
muscles of respiration.
| CAS Number | 51-15-0 |
|---|---|
| Molecular Formula | C7H9ClN2O |
| Molecular Weight | 172.61 |
| InChI Key | HIGSLXSBYYMVKI-UHFFFAOYSA-N |
| Synonyms |
|
Applications:
HPLC Separation of Mixture of Atropine, Atrolactic Acid and Pralidoxime Chloride
April 15, 2019
HPLC Method for Atropine, Atrolactic Acid, Pralidoxime Chloride on Primesep 200 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Atropine, Atrolactic Acid, Pralidoxime Chloride.
Atropine is a tropane alkaloid with the chemical formula C17H23NO3. It is an anticholinergic medication that is used to treat nerve agent, pesticide poisoning, slow heartrate, and is also used to decrease saliva production. Typically, it is administered through injection.
Atrolactic Acid is a 2-hydroxy monocarboxylic acid with the chemical formula C9H10O3. It can cause irritation to eyes, skin, and the respiratory system.
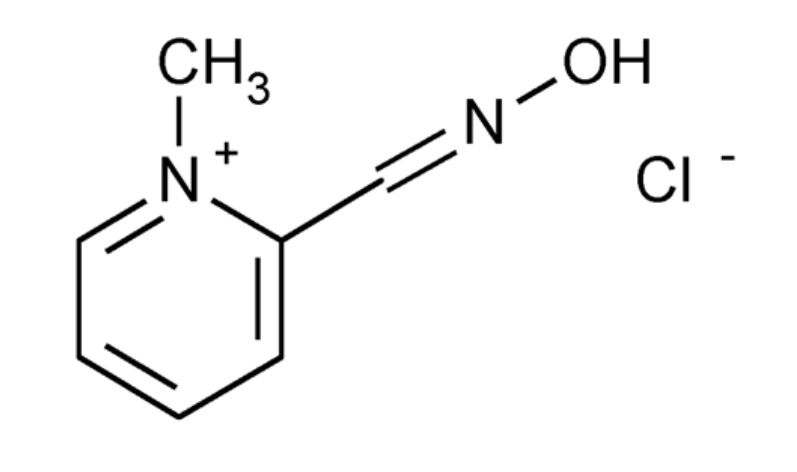
Pralidoxime Chloride, also known as 2-PAM, is a cholinesterase reactivator with the chemical formula C7H9N2O+. It is used as an antidote for poisoning by organophosphate pesticides and nerve agents.
Atropine, Atrolactic Acid, Pralidoxime Chloride can be retained and analyzed using the Primesep 200 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a [buffer] buffer. Detection is performed using UV.
| Column | Primesep 200, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | Gradient H3PO4 – 0.1-0.3%, 10 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Base, Hydrophilic, Hydrophobic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Atropine, Atrolactic Acid, Pralidoxime Chloride |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Atropine
Pralidoxime Chloride

HPLC Determination of Pralidoxime Chloride on Primesep 200 Column
April 15, 2019
HPLC Method for Pralidoxime Chloride on Primesep 200 by SIELC Technologies
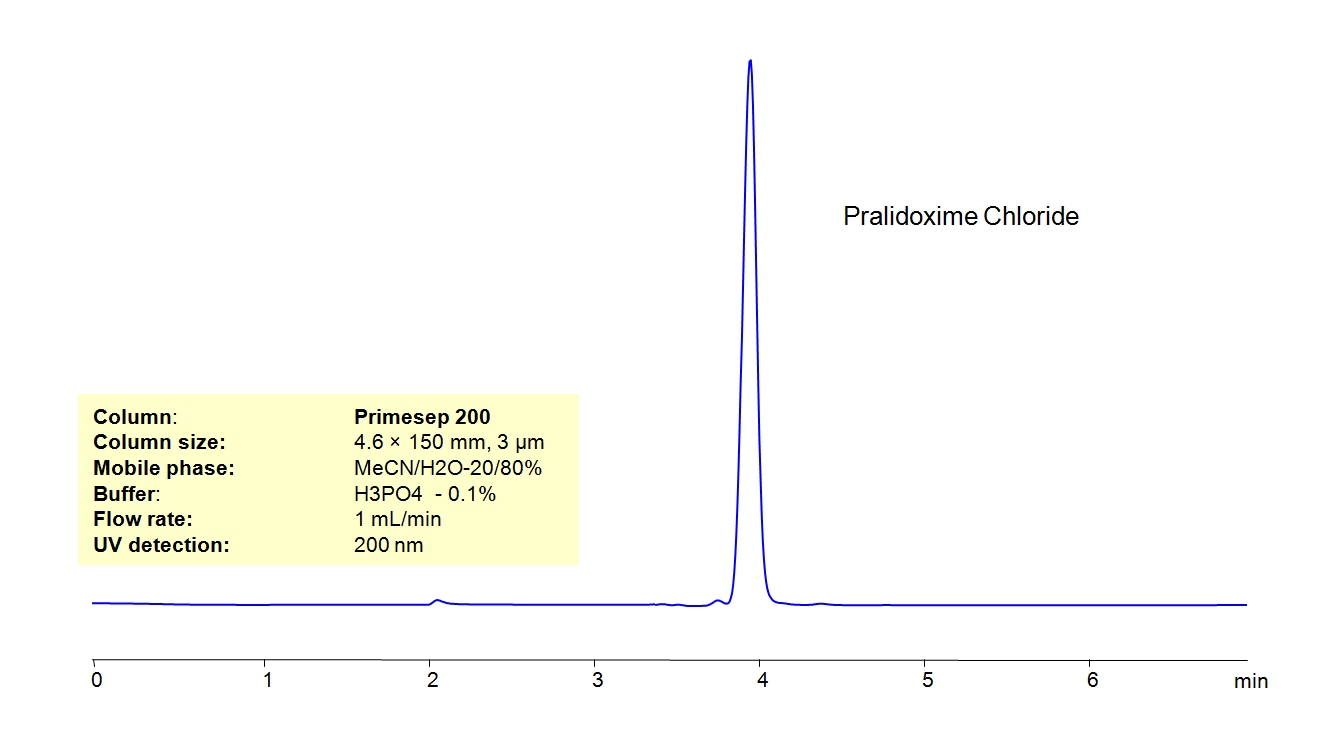
High Performance Liquid Chromatography (HPLC) Method for Analysis of Pralidoxime Chloride.
Pralidoxime Chloride, also known as 2-PAM, is a cholinesterase reactivator with the chemical formula C7H9N2O+. It is used as an antidote for poisoning by organophosphate pesticides and nerve agents.
Pralidoxime Chloride can be retained and analyzed using the Primesep 200 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a [buffer] buffer. Detection is performed using UV.
| Column | Primesep 200, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Basic, Hydrophobic, Ionizable |
| Analyzing Compounds | Pralidoxime Chloride |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended