| CAS Number | 136236-51-6 |
|---|---|
| Molecular Formula | C12H13N |
| Molecular Weight | 171.24 |
| InChI Key | RUOKEQAAGRXIBM-GFCCVEGCSA-N |
| LogP | 1.8 |
| Synonyms |
|
Applications:
HPLC Method for Simultaneous Analysis of Rasagiline and Pramipexole in Tablets on Primesep 100 Column
May 5, 2021
HPLC Method for Pramipexole dihydrochloride monohydrate, Rasagiline on Primesep 100 by SIELC Technologies
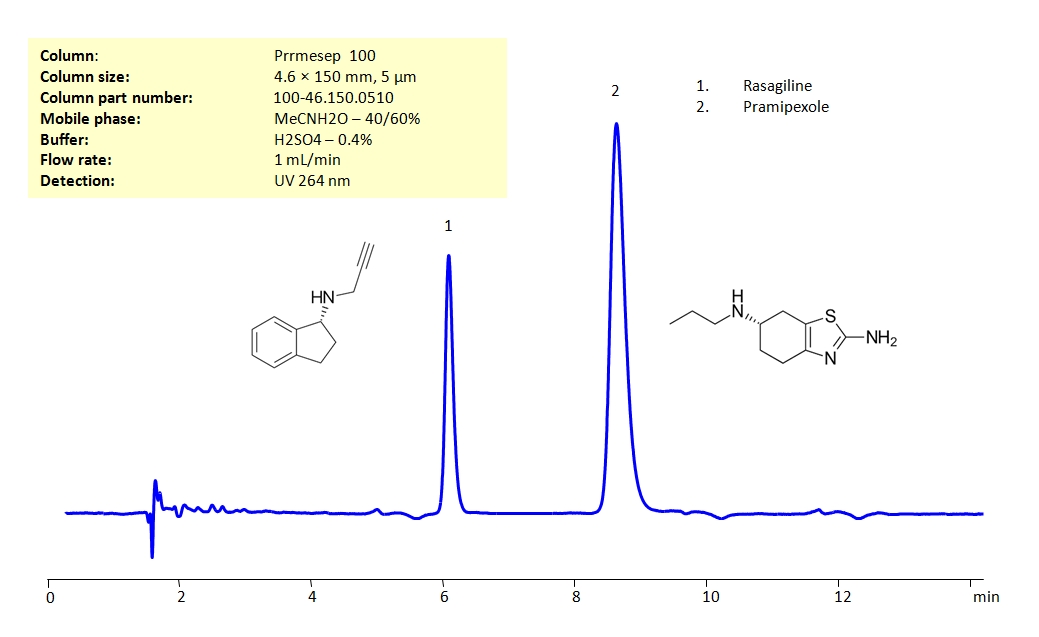
High Performance Liquid Chromatography (HPLC) Method for Analysis of Rasagiline and Pramipexole
Rasagiline and Pramipexole are marvels of modern science, giving patients with Parkin’s disease a way to treat their symptoms. Interestingly, both work slightly differently. Rasagiline prevents dopamine metabolism by permanently binding to the enzyme that breaks down dopamine, monoamine oxidase-B (MOA-B), while pramipexole binds to dopamine receptors and activates them, mimicking dopamine itself.
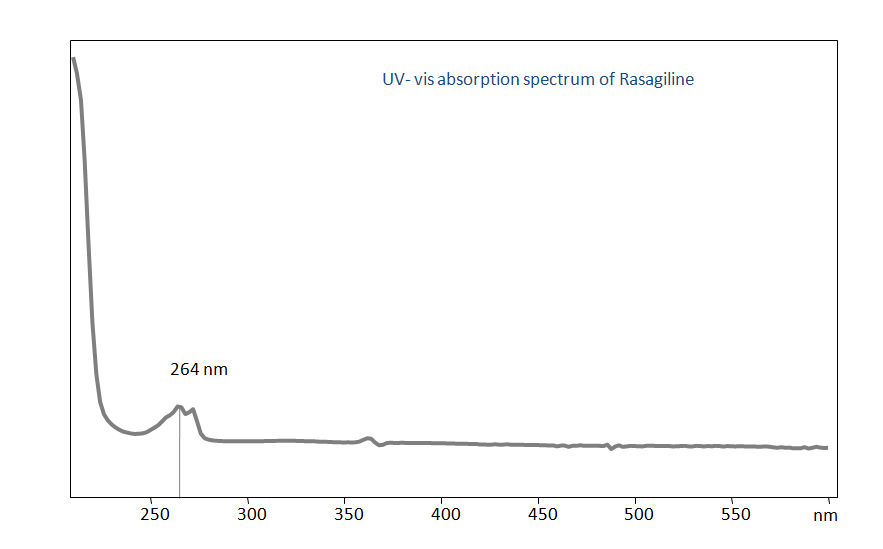
These drugs can be detected in the low UV regime. Using a Primesep 100 reverse-phase column and a mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid (H2SO4) buffer, Rasagiline and Pramipexole can be separated, measured, and analyzed. This analysis method can be UV detected at 264 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | H2SO4 – 0.4% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 264 nm |
| Class of Compounds |
Drug |
| Analyzing Compounds | Pramipexole dihydrochloride monohydrate, Rasagiline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Rasagiline